Comprehensive analysis of solid-state battery technology
2025-02-05
HEXI
Catalogue
-
Definition of solid-state battery
-
Operating mechanism of solid-state batteries
-
Significantly improved energy density
-
Cost considerations
-
Significant advantages of solid-state batteries
Solid-state battery technology: Leading battery innovation with solid electrolytes
Solid-state batteries, a new battery technology, are gradually changing the landscape of the energy sector with their unique advantages. It abandons traditional liquid electrolytes and replaces them with solid electrolytes, a change that brings higher energy density and faster charging speeds. Next, we’ll take a deep dive into what solid-state batteries are, how they work, and how they differ significantly from regular lithium-ion batteries.
Solid-state batteries, as the name suggests, are a type of battery technology that uses solid electrolytes instead of traditional liquid electrolytes. Compared with ordinary lithium-ion batteries, it has higher energy density, faster charging capability, and better safety and durability. Because of this, solid-state batteries have become the research forefront in the field of battery technology.
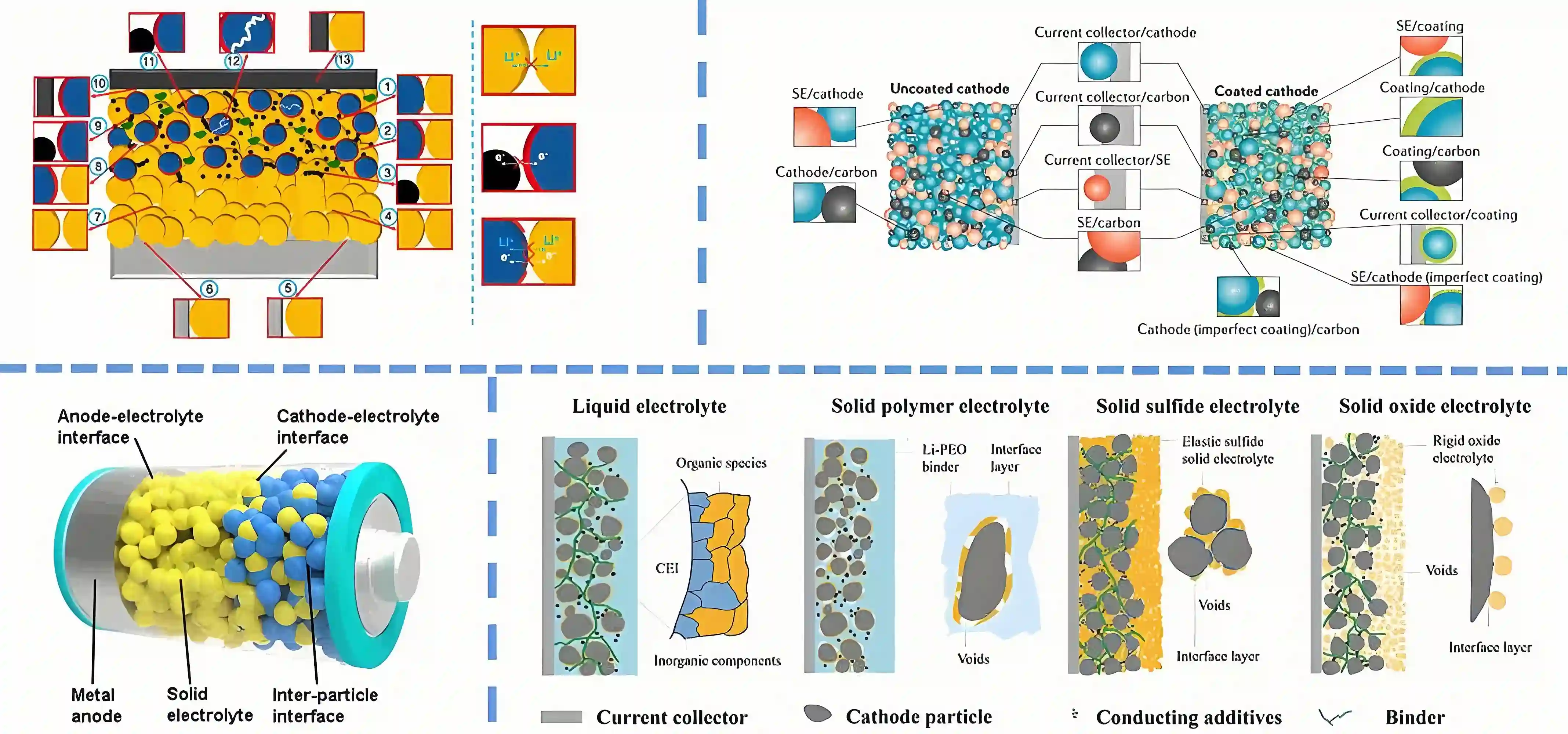
The operating mechanism of solid-state batteries is similar to that of traditional lithium-ion batteries, both of which rely on the movement of lithium ions between positive and negative electrodes and the corresponding transfer of charges. The difference is that the positive and negative electrode materials of solid-state batteries both use solid electrolytes, eliminating the use of liquid electrolytes.
During the charging process, lithium ions migrate from the positive electrode material to the negative electrode material, accompanied by the flow of electrons from the negative electrode to the positive electrode; during discharge, lithium ions migrate in the opposite direction, from the negative electrode back to the positive electrode, and the direction of electron flow is Also changed accordingly.
Compared with traditional lithium-ion batteries, the electrolyte morphology of solid-state batteries has changed significantly. Solid-state batteries abandon liquid electrolytes in favor of solid electrolytes, a change that brings many advantages. First, solid electrolytes exhibit higher ionic conductivity, thereby improving the battery's charging and discharging efficiency. Secondly, its lower interfacial resistance characteristics enable the battery to be charged significantly faster. More importantly, the non-leakage and non-expansion properties of solid electrolytes greatly improve the safety of the battery and also extend its service life. Under high temperature or overcharge conditions, solid-state batteries can remain stable and avoid the risk of explosion or fire, making them particularly suitable for use in fields with extremely high safety requirements such as electric vehicles, aerospace, etc.
Because solid electrolytes have excellent ionic conductivity and low interface resistance, solid-state batteries have higher energy density and faster charging capabilities. Reports show that the energy density of solid-state batteries can be several times higher than that of traditional lithium batteries, and their charging speed is also greatly improved. This makes solid-state batteries ideal for devices such as electric vehicles that require high energy density and fast charging.
Although solid-state batteries have many advantages, their manufacturing costs are currently still relatively high. This is mainly due to the immaturity of
solid-state battery production technology and relatively high production costs. However, with the continuous advancement of technology and process optimization, the cost of solid-state batteries is expected to gradually decrease, thereby promoting their wider application.
Solid-state battery overview
Solid-state batteries are an innovative type of battery. The key is to use solid electrolytes instead of liquid electrolytes in traditional lithium-ion batteries. Traditional liquid lithium batteries are composed of four major components: positive electrode, negative electrode, electrolyte and separator, while solid-state batteries replace them with solid electrolytes. Although the working principles are similar, this key change in solid-state batteries brings significant performance improvements.
Working principle explained
Similar to traditional liquid lithium batteries, the positive and negative electrodes of solid-state batteries achieve the charging and discharging process through the movement of lithium ions. During charging, the lithium ions in the positive electrode are separated from the active material, migrate to the negative electrode through the solid electrolyte, and combine with the electrons migrated from the external circuit at the negative electrode to form lithium atoms or embed them in the negative electrode material. The discharge process is the opposite.
Using solid electrolytes instead of liquid electrolytes not only makes it possible to use positive and negative electrode materials with higher specific capacity, but also fundamentally solves the safety problem of batteries. Therefore, solid-state batteries are seen as an important upgrade direction for lithium-ion batteries.
Solid-state batteries are gradually becoming the leader in the next generation of high-performance lithium batteries due to their high energy density and high safety. In performance comparisons, solid-state batteries have demonstrated significant advantages over liquid batteries in many aspects, including ion conductivity, energy density, high voltage resistance, high temperature resistance, and cycle life. It not only combines the high energy density characteristics of traditional liquid lithium batteries, but also achieves major breakthroughs in safety, making it an ideal choice for electric vehicles and other fields.
(1) Excellent safety
Liquid lithium batteries have the risk of thermal runaway, which can be triggered by adverse factors such as overcharging, impact, short circuit or soaking in water. When the temperature rises to 90°C, the SEI film on the surface of the negative electrode begins to decompose, causing the lithium-embedded carbon to react with the electrolyte to release heat and produce a large amount of flammable gas, which may cause an internal short circuit; when the temperature reaches above 200°C, the gasification and decomposition of the electrolyte will cause the battery to burn violently or even explode.
In contrast, solid-state batteries shine in terms of safety with their five major safety features. First, the high mechanical strength of the solid electrolyte can effectively inhibit the growth of lithium dendrites, thereby reducing the risk of short circuit. Second, its non-flammable and non-explosive properties also greatly reduce safety risks. In addition, solid-state batteries also avoid problems such as continuous interface side reactions, electrolyte leakage and drying, and can maintain stable performance or better in high temperature environments.
(2) High energy density
The energy density of traditional liquid batteries is close to the theoretical limit of 350Wh/kg. However, solid-state batteries can withstand higher voltages (above 5V) and have a wider range of optional materials due to their wide electrochemical window. This allows solid-state batteries to have significant room for improvement in energy density. Since battery energy density is equal to the operating voltage multiplied by the specific capacity, and the overall specific capacity is limited to the lower one of the positive and negative electrodes, the key is to select a negative electrode material with a high specific capacity. At present, the specific capacity of graphite anodes in solid-state batteries is 372mA•h/g, and the theoretical specific capacity of silicon-based anodes and lithium metal anodes is higher, both significantly exceeding the positive electrode materials. This means that solid-state batteries are expected to achieve an energy density of 500Wh/kg or even higher by matching high-specific-capacity anode and cathode materials.
It is worth mentioning that although the research history of solid-state batteries is not short, even earlier than the current liquid battery technology, its commercial mass production still faces many challenges. This undoubtedly increases the difficulty and complexity of its commercialization process. However, with the continuous advancement and breakthroughs in technology, solid-state batteries are expected to gradually achieve commercial mass production and widespread application in the future.
The above picture shows the evolution of lithium battery structure, with traditional liquid batteries on the left and solid-state batteries on the right. You may be quite familiar with the structure of liquid batteries, which contain positive and negative electrodes and electrolytes in the middle, separated by diaphragms. However, this structure has an inherent problem: although the diaphragm is intended to play a protective role, it is extremely challenging to ensure its absolute safety. This is mainly because the diaphragm needs to meet the two requirements of allowing electrons to pass through and isolating the electrolyte at the same time, which undoubtedly increases the risk of its breakdown.
